pH has a big impact on the performance of bubble juice -- at least those made from dishwashing liquid. The optimal pH depends both on the particular detergent and the polymers. Some detergents are much more sensitive to pH differences than others.
pH is a measure of water-based solution's acidity or basicity. It is an important factor in making quality bubble juice. Ingredients such as baking powder, citric acid, baking soda, and vinegar are often used to tune a bubble juice's pH into the optimal range. For technical information about pH, see the Wikipedia article. In addition to the material below, see the pH Adjusters and Water Conditioners article for information about ingredients use to adjust the pH.

Getting bubble juice pH right has many benefits. When the pH is optimal, bubbles tend to be stronger, easier to close and live longer than with untuned bubble juice. Depending on the detergent, optimizing the pH may also have a dramatic impact on the detergent's efficiency allowing less detergent (sometimes much less) to be used to achieve the same film thickness. The difference is often quite dramatic. In some cases, the difference is subtle but distinct. The optimal pH range depends on the particular detergent as well as the polymers.
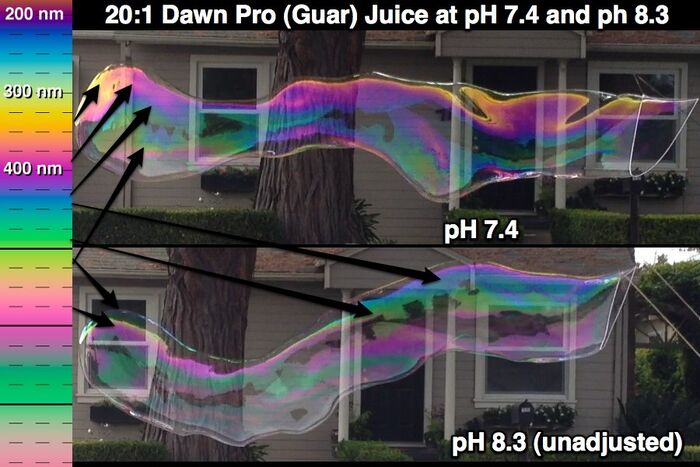
Distinct effects at different pHs. With Dawn, Fairy and related detergents, there are two distinct effects that kick in at different pH values. For dilute solutions (let's say 16 or 18 to 1 or more dilute), there is a distinct improvement in bubble longevity and the ease with which large bubbles can be closed somewhere around pH 8.3. Unadjusted bubble juice will usually have a pH of 9.0-9.2 (depending somewhat on the water used). When the pH falls below about 7.6 (it could be somewhat higher), there is a distinct improvement in the detergent efficiency which results in a thinner (as evidenced by the color profile) but stronger (as evidenced by longevity) film than unadjusted juice.
Some detergents (such as Japan's Charmy) become dramatically more effective when their pH is tuned. In some cases, tuning the pH can triple the detergent's effectiveness, allowing you to use 1/3 the amount needed for an untuned juice.
Though there are exceptions, most commercial dishwashing liquids we've tested have a pH well over 8.1 when combined with tap or distilled water. Dawn Pro (and others in the Dawn family that we have tried) and distilled water has a pH of about 9.1 at a fairly large range of dilutions. Most Procter & Gamble detergents (such as Dawn, Fairy and Dreft) and Japan's Charmy, seem to work best when the bubble juice has a pH in the range of 7.2 - 7.8. A little bit higher or slightly lower, may also work well. At this time (Aug 2018), the range is not strongly defined in the sense that a Dawn-based recipe with a pH of 7.2 does not seem to perform differently from the same recipe adjusted to 7.8. There seems to be fairly strong consensus that this range works well. Preliminary experiments to further refine the pH range have been inconclusive.
pH and dilution. There are some surprises when diluting many detergents. When you add distilled water (which has of pH 5.5-7.0 depending on its exposure to air) to pure Dawn Pro (or similar) the pH rises from about 8.5 to about 9.1 at a dilution of 20:1. This explains why pH adjustment is so much more critical for dilute solutions (above about 16 or 18 to 1) than for core concentrated solutions. Aug 2014 NOTE: We are currently exploring the reasons for this behavior and mapping dilution to pH to see if it yields information that we can use in bubble brewing and comparing detergents).
The value of pH adjustment. Optimizing a juice's pH can have a dramatic impact on juice with some dilutions and in some conditions. In other conditions, the difference may not be so pronounced. Some recent tests on a cool humid (80% RH) morning showed subtle differences between juice at ph 7.4 and 9.0 (which is the normal pH of water and Dawn Pro at 20:1). In the early evening, when humidity was just over 50%, the difference in performance was dramatic with the pH-adjusted bubbles being much easier to form and lasting much longer (on average) than the unadjusted juice. The impact of pH-adjustment is much more dramatic with solutions that are somewhat dilute (with Dawn Pro the impact goes up substantially when the dilution is 16:1 or greater).
Dependencies. The optimal pH will depend on both the detergent being used and the polymer. While most bubble polymers seem to work well in a broad range of pHs, Japanese PVA laundry starch-based mixes do not work well when the pH is lowered (at least with baking powder).
Outside the range. For Dawn/Fairy-based juice, the optimal range seems to be about 7.2 - 7.8. Dawn/Fairy juice with a pH up to about 8.2 also works well although when the pH is over about 7.8 you lose the obvious impact on detergent efficiency (as evidenced by the color profile). The effect on film thickness (efficiency) seems to kick in somewhere in the 7.8 range and does not change noticeably as the pH drops more. Juice can work quite well in the low 8's. Performance degrades somewhere over 8.3. Dawn-based juice with a low pH (let's say 6.7-6.8) can work better than unadjusted juice, but if the pH goes lower, the juice may not work at all. Juice with a pH below 7 seems not quite as friendly as when the pH is above 7.0.
PH RANGE NOTE (March 2014). Some casual testing seems to indicate that Dawn Pro-based bubble juice was usable at pH 6.8 but performed less well than juice that had a pH of about 7.4. Also, juice with a pH of about 7.9 performed less well than the pH 7.4 juice. Ghosting in the pH 7.9 juice was weaker than with the 7.4 juice. It is not clear whether the pH difference or the alkalinity difference was the primary factor related to the ghosting.
Gentle pH Adjustment (CO2 method)[]
The carbon dioxide given off when some ingredients are mixed with water turns out to be a gentle and effective method for adjusting the pH of bubble juice. For years, people had noticed that these ingredients improved bubble juice but there was a lot of debate as to why. It is now clear that the carbon dioxide given off when baking powder or baking soda+citric acid are mixed with water gently lowers the pH of bubble juice into a range that improves the juice's performance.
Some of the carbon dioxide dissolves in the water creating carbonic acid, a weak acid. Because water can only hold a small amount of carbon dioxide, it is very hard to over-acidify the mix when using baking powder or baking soda+citric acid or baking soda+tartaric acid (cream of tartar). When using baking soda+citric or tartaric acid, it is important to use the soda and acid in the correct balance; otherwise, the solution might become too acidic or not acidic enough.
Mixing order. Because carbon dioxide is not very soluble in water (and not very soluble in very warm and hot water), it must be introduced when all the water (and preferably the detergent also) is present. If you are using baking soda + citric acid, you can use one of those ingredients (usually the baking soda) early in the process and add the other after all other ingredients are present. There is often a benefit to using the baking soda when adding the polymer. So, people often use baking soda as the first ingredient and add the citric acid last. If using baking powder, it is best to add it as the very last ingredient.
Water temperature. Because carbon dioxide is not very soluble in hot water, it is best for the carbon dioxide to be created when the water is near room temperature (or colder). Slightly warm water is ok, but if the CO2 is created when the water is very warm or hot, the pH may not be lowered enough to be beneficial.
Amounts. 1/2 - 1 tsp (2.5 - 5 ml) baking powder (single-acting is best but double-acting will work but may take a day or two to complete its reaction) per liter of water is effective for most water -- including distilled water (which is slightly acidic due to dissolved atmospheric carbon dioxide if it has been exposed to air). 1 to 2 grams anhydrous baking soda plus half that amount of citric acid can also be effective. While a 2:1 dry baking soda:citric acid ratio is often effective, we have received reports (late Spring/early Summer 2014) from people that have had result that were more acidic than expected. We are actively investigating this issue (July/August 2014) and hope to have an update by September. If you do not have a pH meter, we recommend using baking powder.
Concentrates. Since the creation of the C02 needs to be done when all the juice's water is present, baking powder or baking soda+citric acid are not very effective for adjusting pH when making concentrates. In most cases, if a concentrate contains baking powder or baking soda+citric acid, more baking powder (or baking soda+citric acid) should be added after dilution.
Other methods. There are other methods of adjusting pH, too. Citric acid or tartaric acid or distilled vinegar can be used. Carbonated water can also be used. When using acids directly, care must be taken not to overacidify the mix. Bubble juice tends not to work at all if the pH is too low. The desired pH depends on the detergent. If using an acid directly (such as citric acid or tartaric acid or vinegar), it is best to use a pH meter to make sure that you don't over-acidify the mix. The amount of acid needed will depend on the pH and alkalinity of your water. Edward Spiegel notes that for a 20:1 water:detergent mix about 1.5-2 tsp (7.5-10.0 ml) of distilled vinegar per liter water (not bubble juice) achieves a pH of 7.1-7.6 with his pH 9.0 tap water. As little as 0.25 grams citric acid has a similar effect.
Film Thickness and Other Factors[]
The most visible impact of pH adjustment is on the film thickness which is responsible for the bubble colors and bubble juice's "color profile". To see this effect, you need to examine the bubbles in conditions that allow you to accurately see their colors.
At any given dilution near or above the critical dilution, pH-adjusted juice will create bubbles with thinner films than unadjusted bubble juice. In other words, it will behave as if there is more detergent than there is. Though the pH-optimized juice will create thinner films than an unadjusted juice with the same dilution, the film is generally stronger with better longevity than the unadjusted juice's.
ph Adjustment and dilute solutions. pH adjustment has a less noticeable impact on bubbles juice that has a relatively large amount of detergent (with Dawn-based mixes that would be solutions that have a water-detergent ratio of about 15:1 or lower). With more dilute solutions, the difference can be quite remarkable in terms of bubble-friendliness (ease of closing bubbles) and/or longevity. In one series of tests in very low humidity, Wayne Schmidt reported: "The solution without the baking powder produced 30 bubbles that lasted an average of 6.5 seconds. 73-percent of the attempts produced bubbles that closed. The solution with baking powder produced 32 bubbles that lasted an average of 11.1 seconds and had a closure rate of 94-percent."
Ghosting[]
pH can influence the ghosting of juice that tends to create ghosts (such as juice made with Procter&Gamble detergents or other detergents that use Sodium X Sulfate). When the pH is in the optimal range, ghosting tends to be heavier than when the pH is above the optimal range. Some people reduce ghosting by increasing the alkalinity of a mix, but this often makes the bubble juice less effective though it can still work quite well.
pH and Polymers[]
While most polymers and other helpers used for bubble juice seem to be effective in a broad pH range, some appear to have more restricted pH ranges. For example, Japanese PVA-containing laundry starch seems to perform poorly when baking powder is present (although it is not certain that this is a pH issue).
Odds and Ends[]
Milky/cloudy appearance after adjustment. When you adjust the pH of juice made with some detergents, the bubble juice may become slightly milky or cloudy. This is true of when the pH falls below about 8 with Dawn and related detergents (such as Fairy) and possibly others that are based on the Sodium X Sulfate family of surfactants. This change in appearance appears not to be strictly pH-related and may have to do with the manner used to adjust the pH. An experiment using C02 only to adjust pH (by force carbonating the dilution water) indicated that the appearance went from cloudy to clear as the pH rose (as the excess carbon dioxide outgassed over a few days).
Using vinegar. Distilled vinegar (pH about 2.5) can be useful, but care must be taken not to overacidify the mix. About 2.5 ml (1/2 teaspoon) distilled white vinegar added to 500 ml bubble juice with a pH around 9.0/9.1 will bring the pH down to about 7.5-7.7 in most cases. If adding the vinegar causes the quality of the juice to decline, you can add a little baking soda to improve the juice (by raising its pH). NOTE (Aug 2014): Juice whose pH is adjusted with vinegar may not perform as well as juice adjusted with baking powder or citric acid or baking soda/citric acid. More research is being done.
Measuring pH[]
There are many ways to measure pH. For bubble juice, it is useful to have something that can measure a pretty good range of pH (at the very least from 5-10 but it can be useful to have something that can measure as low as 2). There are inexpensive pH meters that work well-enough for bubble chemistry as long as they are occasionally calibrated (more on this below) and are frequently checked for reasonableness. There are a great many misunderstandings about pH due to people publishing conclusions based on either faulty or uncalibrated pH devices.
Dye-based pool pH-measuring kits are generally not very helpful -- both because they are designed to test a pretty narrow range of pH and because bubble juice often contains detergents whose dyes make it difficult to accurately read the results.
pH paper can be ok, but there is a wide variation of quality AND quality may decline with age. Be sure to check the accuracy of the paper before drawing any conclusions by using it to test a known buffer solution.
Electronic soil ph-measuring kits are not appropriate. They are specially-designed for measuring the pH of soil and can be quite far off when measuring the pH of liquids.
Buffer solutions. Buffer solutions are sold for the purpose of testing and calibrating pH measuring devices. They are quite inexpensive and can save you much grief. Some pH meters need particular pHs for calibration. It is worthwhile to have both an acidic and a basic buffer on hand to get a sense of how true the pH meter is. I purchase single use buffer solutions in foil packets and try to have a supply of 4.0, 7.0 and 10.0 packets.
Checking calibration. Any time that you are taking measurements that matter, it is worth checking your pH meter with either a buffer solution or a stable solution whose pH you have established. I find Dawn Pro and distilled vinegar to be good test solutions as their pHs are pretty stable. We are not too worried about being off by 0.1 or 0.2, but many people accidentally have reported results based on meters giving either erratic results (common when the batteries are dying or if the meter is faulty) or that have never been calibrated. Calibrate your device then take measurements of your high-quality detergent and a high-quality distilled vinegar and make a note of their values. Whenever you are taking critical readings, test your device with your detergent and some vinegar to make sure that the readings seem reasonable.
See Also[]
pH Adjusters and Water Conditioners article
ph Basics and FAQ (Coming soon)