(add note about stability of index of refraction) |
(→Dilution and Color: improve clarity of the presentation) |
||
| Line 11: | Line 11: | ||
[[File:Thommy_dome_for_soapfilmthickness_IMG_0966.JPG|thumb|600px|left|Bubble dome picture by Thommy]]{{ClearFloat}} |
[[File:Thommy_dome_for_soapfilmthickness_IMG_0966.JPG|thumb|600px|left|Bubble dome picture by Thommy]]{{ClearFloat}} |
||
==Dilution and Color== |
==Dilution and Color== |
||
| + | Dilution can have a big impact on bubble colors because of the way that dilution influences the soap film thickness. These color variations are especially noticeable with medium and big bubbles. |
||
| − | The detergent concentration can have a big impact on the color because of its influence on the surface tension which in turn influences the film thickness. Very dilute detergent solutions whose surfactant concentration is in the neighborhood of the CMC (Critical Micelle Concentration) have film thicknesses more heavily influenced by the amount of detergent than solutions where the detergent concentration is higher than the CMC. The dilution ratios where this occurs varies from detergent to detergent. |
||
| + | |||
| + | '''Very very dilute''' solutions create very thick films may even be colorless and glossy -- as opposed to very very thin film that are colorless and glossles and almost invisible. '''Very dilute solutions''' (where the surfactant concentration is low) have '''thick''' films which result in films dominate by shades of green and red. '''As the dilution becomes less extreme''', the greens, reds and pinks become more saturated and then more purples, yellows and blue start to appear. Soon after solutions exceed something callled the [[Critical Micelle Concentration]] (CMC), increasing the concentration has little or no impact on the colors. |
||
| + | |||
| + | These color changes are related to changes in the solution surface tension. Due to the way that surfactants work, these changes occur primarily in the neighborhood of the CMC. |
||
| + | The dilution ratios where this occurs varies (sometimes considerably) from detergent to detergent. The CMC also seems to be influenced by the pH for some detergents (such as [[Charmy Dish Detergent]] ). |
||
The images below are of similar-sized bubbles created with PEO-based bubble juice whose only difference was the soap dilution. |
The images below are of similar-sized bubbles created with PEO-based bubble juice whose only difference was the soap dilution. |
||
Revision as of 00:18, 4 August 2013
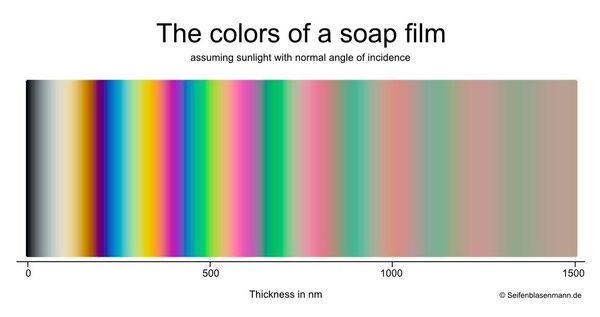
Created by Bjorn of seifenblasenmann.de and used with his permission
A soap bubble's colors reveal a great deal about the soap film. Because of the way that the colors are created, the colors correspond to the thickness of the film. These colors can provide very useful information. For instance, when creating a large bubble, you can often see large portions of the bubble turn from blue to amber and then start to become transparent. These colors indicate that the film is becoming thin -- and it is probably time to close the bubble.
The colors can also help determine the optimal dilution for a water:detergent mix. When there is a lot of excess soap in the mix, the surface tension will be relatively low which allows the film to be thin. In this dilution range, bubbles will show the range of colors seen on the left portion of the chart. If enough water is added that there is little excess soap (the critical micelle concentration , or CMC, has been approached), bubbles will tend to show the muted (but quite beautiful) pinks and greens seen at the right side of the chart.
There are a few charts which map color to thickness. There is some disagreement (usually small) between the different available charts due to slightly different assumptions about the parameters such as index of refraction, viewing angle, etc.
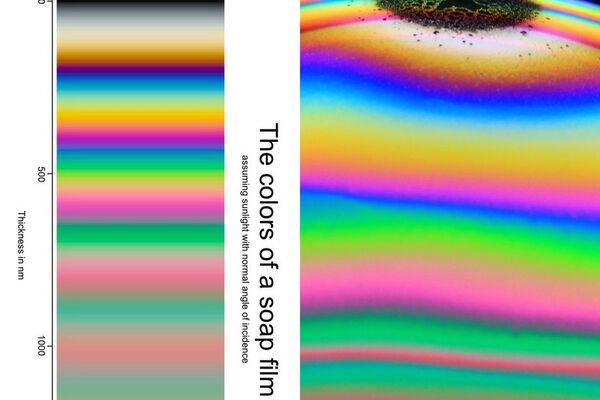
At left, Bjorn's chart which was generated by theory. Right, a bubble dome section illustrating. Photo by Thomas Poersch.

Bubble dome picture by Thommy
Dilution and Color
Dilution can have a big impact on bubble colors because of the way that dilution influences the soap film thickness. These color variations are especially noticeable with medium and big bubbles.
Very very dilute solutions create very thick films may even be colorless and glossy -- as opposed to very very thin film that are colorless and glossles and almost invisible. Very dilute solutions (where the surfactant concentration is low) have thick films which result in films dominate by shades of green and red. As the dilution becomes less extreme, the greens, reds and pinks become more saturated and then more purples, yellows and blue start to appear. Soon after solutions exceed something callled the Critical Micelle Concentration (CMC), increasing the concentration has little or no impact on the colors.
These color changes are related to changes in the solution surface tension. Due to the way that surfactants work, these changes occur primarily in the neighborhood of the CMC. The dilution ratios where this occurs varies (sometimes considerably) from detergent to detergent. The CMC also seems to be influenced by the pH for some detergents (such as Charmy Dish Detergent ).
The images below are of similar-sized bubbles created with PEO-based bubble juice whose only difference was the soap dilution.
More Examples

Rose and green dominate when the water:detergent ratio is high. This bubble was made with Dawn Pro at a dilution of about 45:1 (water:detergent).

Note the pink/green shades typical of very dilute solutions.

This bubble was created with bubble juice that has a 24:1 Water:Dawn Pro ratio
Thank you to Bjorn of Seifenblasenmann.defor the use of his chart.
Index of Refraction
Some question has been raised as to whether the assumptions about the relationship between color and film thickness are influenced by the components of the bubble juice. A physicist specializing in optics gave some thought to the problem and expressed confidence that the difference in the index of refraction of mixes would not vary enough to have significant impact on the relationship.



